Cortisol: The Stress Hormone
Cortisol, often called the body’s “stress hormone,” is produced by the adrenal cortex, which is the outer layer of the adrenal glands located above the kidneys. It belongs to a class of steroid hormones known as glucocorticoids, which help regulate metabolism, control immune responses, and shape how the body responds to stress. When you are startled awake by a 3 A.M. crisis call or receive alarming news mid-day, cortisol is one of the key hormones orchestrating the body’s reaction. However, cortisol’s influence extends far beyond emergency “fight-or-flight” responses. It helps regulate metabolism, immune function, blood pressure, and even memory processes. In this article, we will explore how cortisol is regulated via the hypothalamic-pituitary-adrenal (HPA) axis, its daily rhythm in the bloodstream, and the difference between adaptive short-term stress responses and harmful chronic exposure. Building on this understanding of cortisol, leaders can better interpret their own physical responses under pressure and lay the groundwork for the more complex interactions we’ll discuss in later articles.

The HPA Axis: How Stress Signals Trigger Cortisol Release
When you experience a stressor, say, an unexpected financial shock hitting an organization, your brain activates a cascade known as the hypothalamic-pituitary-adrenal axis (HPA axis). In the HPA axis, the hypothalamus (specifically the paraventricular nucleus, PVN) secretes corticotropin-releasing hormone (CRH), which signals the pituitary gland to release adrenocorticotropic hormone (ACTH) into the bloodstream. ACTH then travels to the adrenal glands (located atop the kidneys) and stimulates the adrenal cortex to produce cortisol. This entire sequence can unfold within minutes of a perceived threat. Importantly, cortisol release is part of a broader coordinated stress response that also involves the sympathetic nervous system; while cortisol is surging via the HPA axis, the adrenal medulla pumps out adrenaline and noradrenaline to kickstart the immediate “fight-or-flight” response. Adrenaline works within seconds (elevating heart rate and blood pressure), whereas cortisol typically peaks a bit later and helps sustain the body’s alertness and energy supply.

Cortisol’s effects are widespread because virtually every tissue in the body has receptors for it. In a stressful meeting, for example, rising cortisol will mobilize glucose into the bloodstream for quick energy, temporarily sharpen memory for the salient threat, and dampen non-essential functions (like digestion or immune responses) to prioritize immediate survival needs. You can consider cortisol as the body’s emergency manager: when a crisis strikes, it rapidly reallocates resources, boosting functions critical to handling the immediate threat while putting less urgent operations on hold.
These acute actions of cortisol are highly adaptive, helping you meet sudden challenges effectively. Equally important, however, is shutting down the emergency response once the challenge passes. The body relies on a negative feedback loop: elevated cortisol signals the brain to reduce production of CRH and ACTH, which in turn curbs further cortisol release. This self-regulating mechanism prevents cortisol from over-accumulating when it’s no longer needed. In an optimal scenario, this feedback ensures cortisol spikes are brief—like a manager calling off the emergency team once the crisis is contained—so the body can return to baseline, a state of equilibrium known as homeostasis.
Most cortisol circulating in the bloodstream is bound to carrier proteins, with only a small fraction remaining “free” and biologically active at any given time. Salivary cortisol measurement, which will be referenced throughout this series on cortisol and leaders’ decision-making, specifically captures this free fraction. As a result, it has become a widely used, noninvasive method for assessing HPA axis activity in research. For example, if your salivary cortisol is sampled during a high-pressure simulation, you will typically see your cortisol level rise in response to the acute stressor, which reflects the activation of your HPA axis. This increase indicates your body’s mobilization of resources to meet the perceived challenge.
Circadian Rhythm and the Cortisol Awakening Response (CAR)
Cortisol is not only reactive but also rhythmic. Even on a normal day with no acute stressors, cortisol levels in the body follow a strong 24-hour cycle governed by the body’s internal clock (the circadian rhythm). Under normal conditions, cortisol levels begin to rise in the second half of the night, peaking in the early morning shortly after waking, then gradually decline throughout the day to very low levels in the late evening. By midnight, when one is typically asleep, cortisol is at its daily nadir (minimum), and then the cycle begins anew before dawn. This built-in rhythm is thought to help jump-start our metabolism and alertness in the morning (when we face the “stress” of starting a new day) and allow for rest and recovery at night.
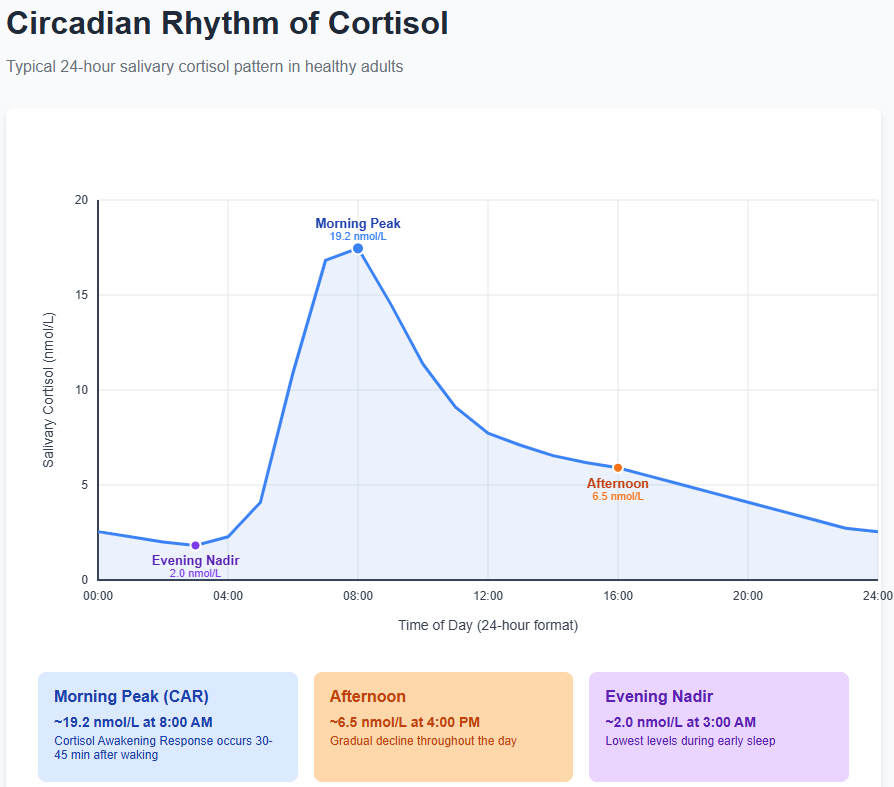
A notable feature of this cycle is the cortisol awakening response (CAR), which is the rapid rise (about 50–60%) in cortisol levels that occurs within the first 30–45 minutes after awakening in the morning. Think of the CAR as your body’s natural boost of energy and alertness to get you going. This morning peak is especially advantageous for tackling complex or demanding tasks that require focus, problem-solving, or emotional regulation. Under healthy conditions, after this post-awakening spike, cortisol follows a diurnal slope—a steady downward trend in cortisol levels from morning to evening that reflects the body’s natural rhythm of stress hormone regulation. By afternoon and evening, cortisol is much lower than in the morning, helping the body wind down toward sleep.
Why is this important for leaders? Research shows that the pattern of cortisol across the day can be an indicator of how the body is handling chronic stress. A robust CAR and a steep diurnal decline (high in the morning, low at night) are generally a sign of a well-functioning HPA axis. By contrast, chronic stress is often associated with a flattening of the diurnal slope. This means the gap between high morning and low evening cortisol narrows. Sometimes chronically stressed individuals even show a blunted CAR (not much morning rise) or elevated evening cortisol, both of which can be red flags. For example, you have been under unrelenting job strain and might start to feel “tired but wired” at night. This is a possible sign that your cortisol remains abnormally high later in the day when it should be dropping. Over time, such patterns can portend health issues (as we’ll explore shortly).
Measuring cortisol can be tricky and highly context-dependent. A single blood or saliva sample tells you what cortisol is doing at that moment, but without the context of time of day and recent events, it’s difficult to interpret. A cortisol level that is high at 7 A.M. might be perfectly normal (part of the CAR), whereas the same level at 11 P.M. would be unusually elevated. Thus, when you undergo health check-ups that include cortisol, it’s important to interpret those values in context. Many research studies collect cortisol multiple times a day over a period (e.g., samples at wake-up, 30 minutes after wake-up, afternoon, and bedtime) to map the curve. Newer methods even analyze hair cortisol, which accumulates cortisol over weeks, providing an index of long-term cortisol exposure (imagine a strand of hair preserving a timeline of stress). These methods are helping researchers and practitioners get a fuller picture of a leader’s stress profile beyond one-off measurements.
Acute Stress vs. Chronic Stress: Adaptation and Allostatic Load
Cortisol’s dual nature means it can be both protective and harmful, depending on the context. In acute stress (short-term, immediate threats), cortisol is part of the adaptive “fight-or-flight” arsenal that can save your life or help you think on your feet. For instance, if a nonprofit director has to suddenly deal with a major donor’s public relations crisis, a spike of cortisol can mobilize glucose to fuel the brain, sharpen attention on the problem at hand, and even create a memory of the event (so it can be avoided or managed better next time). In the short run, these are useful cognitive and physiological adjustments. Cortisol helps you adapt to challenges. Notably, cortisol’s effects come in two waves: a fast, non-genomic effect (within minutes, which influences neurotransmitters and acute metabolism) and a slower, genomic effect (over hours, changing gene expression in cells). The fast effects help you react now; the slower ones help rebalance the system and repair afterward.
Problems arise when stress is chronic. When the HPA axis is activated repeatedly or remains activated for too long without sufficient recovery. Allostatic load is the term scientists use to describe the cumulative “wear and tear” on the body’s systems due to chronic stress and elevated stress mediators like cortisol (the term “allostasis” means achieving stability through change, essentially the process of the body adjusting to stress; allostatic load is the price of repeated adjustments). Under conditions of unrelenting pressure, for example, a city hospital administrator facing crisis after crisis during a prolonged pandemic, the finely tuned stress-response system can start to malfunction. Cortisol levels might remain persistently elevated, or, paradoxically, the adrenal glands might start to “burn out” and produce insufficient cortisol at times due to dysregulation. Both scenarios are problematic. Chronically high cortisol can contribute to hypertension, impaired immune responses, glucose intolerance, and even changes in brain structure (e.g., shrinking of certain hippocampal neurons). In fact, long-term cortisol exposure is linked to a broad range of health issues, including metabolic syndrome, obesity, type 2 diabetes, cardiovascular disease, anxiety, depression, and memory decline. On the other hand, an abnormally low cortisol output (sometimes seen in burnout or PTSD) can also be unhealthy, associated with fatigue, inflammation, and pain syndromes. The ideal is a flexible system: one that produces enough cortisol when needed and turns it off when not, like a thermostat that keeps the room at the right temperature.
Here is a Day in the Life of Cortisol. On a normal (non-crisis) workday, around 6:00 a.m., your cortisol levels are climbing sharply. This is the CAR doing its job, which helps you wake up with energy to tackle the morning routine. You feel relatively alert as you review briefing notes on the bus ride to school. At 8:00 a.m., during a tense staff meeting about upcoming budget cuts, you encounter your first acute stressor of the day. Within minutes, your HPA axis responds: by 8:20 a.m., cortisol in your saliva is measurably higher than at wake-up. You notice feeling a bit “on edge” but also mentally sharp, as cortisol and adrenaline together are enhancing your focus and reflexes. By late morning, the stress of the meeting has passed; you take a brief coffee break, and your cortisol starts to subside as feedback signals in your brain kick in to curb further release. In the afternoon, you deal with routine issues (your cortisol following its natural downward slope). However, at 3:00 p.m., an urgent email from your supervisor about a compliance issue triggers another HPA spike. It’s not as high as the morning surge, as the stressor is moderate, but it’s enough that you feel a flush of heat and a touch of irritability (classic cortisol effects along with sympathetic activation). You handle the issue, perhaps even performing better because the stress sharpens your cognition, and by early evening your hormone levels are trending down again. Now, importantly, if you can disconnect in the evening, such as have dinner with family, maybe exercise, or unwind, your cortisol should bottom out by midnight, which allows your body and brain to recover. If you ruminate all night about work, though, your HPA axis might stay in a low simmer, which causes fitful sleep and a higher baseline come morning. Multiply that scenario by weeks or months, and you see how chronic stress can build up allostatic load.
In summary, cortisol is a cornerstone of our stress response system, neither good nor bad in itself. It is incredibly useful in the right doses at the right times and potentially damaging when it’s chronically out of balance. Leaders who are equipped with this understanding can move beyond the simplistic “stress is bad” mindset to a strategic view: stress can be leveraged and managed. The following articles in this series will focus on how cortisol specifically influences cognition and decision-making, the paradoxes of stress in leadership roles, regulation of emotion under stress, the toll of chronic stress, ways to manage and mitigate harmful stress, gender differences, and social and ethical dimensions of stress. With the foundation laid, we now turn to the immediate impacts of cortisol on how leaders think and make decisions.


